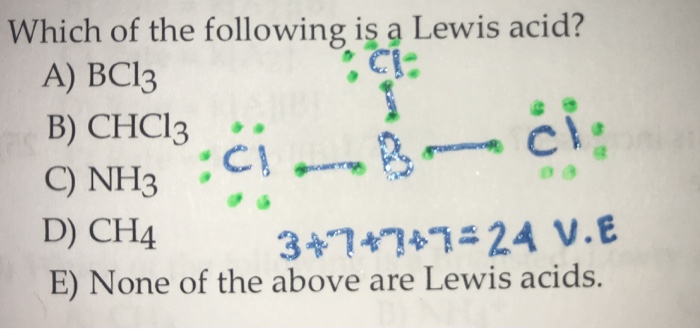
Decide whether the given substance should be classified as a Lewis acid or a Lewis base.BCl3 (Hint; Draw the Lewis dot structure.) | Homework.Study.com

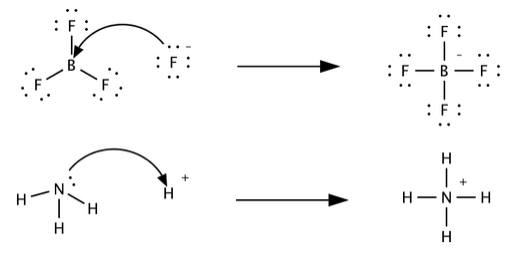
SOLVED: Identify the Lewis acids and Lewis bases: BCl3 +Ci = BCIA" The Lewis base is CI^ The Lewis acid is BCI3 SO2 + H20 = H2803 The Lewis base is H2O
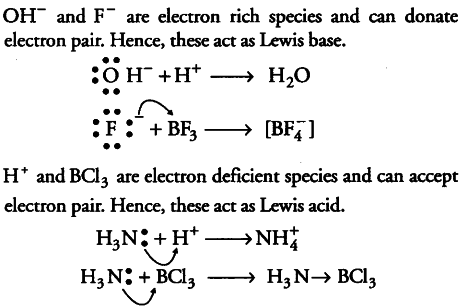
Classify the following species into Lewis acids and Lewis bases and show how these act as Lewis acid/base? - CBSE Class 11 Chemistry - Learn CBSE Forum

Classify the following species into Lewis acids and Lewis bases and show how these act as Lewis ... - YouTube
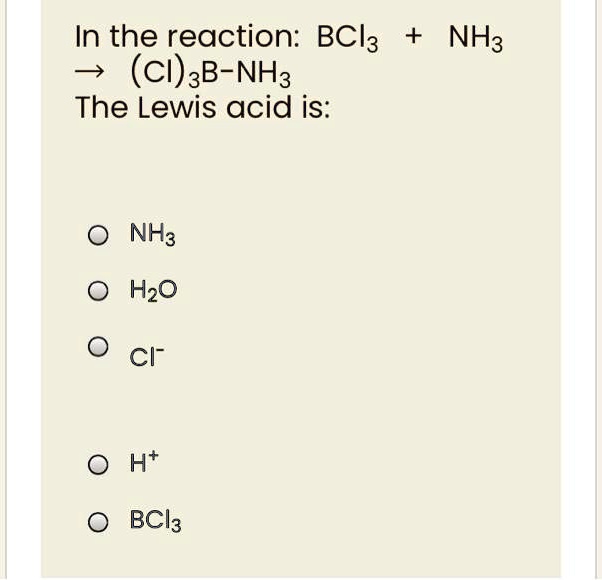
Draw the structures of BCl3.NH3 and AlCl3 (dimer). - Sarthaks eConnect | Largest Online Education Community

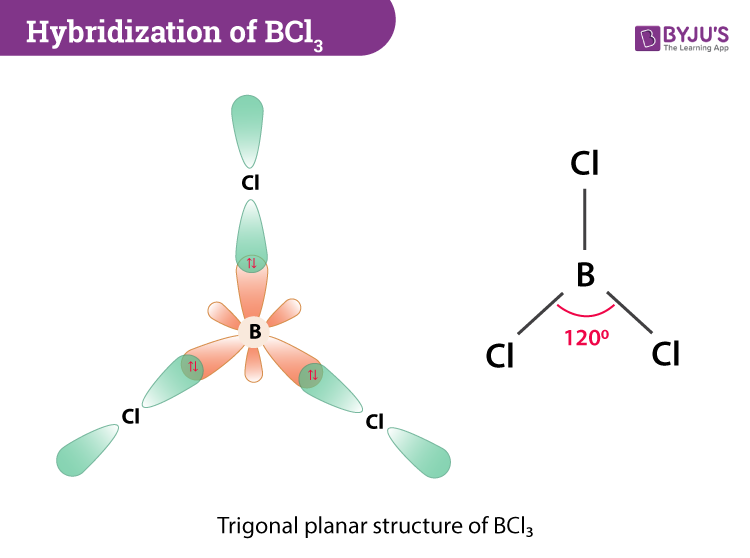
BCl3 lewis structure, molecular geometry, polar or nonpolar, hybridization, Bond angle in 2022 | Molecular geometry, Molecular, Vsepr theory

The Lewis acid character of boron trihalides decreases as: `B Br_(3) gt BCl_(3) gt BF_(3)`. - YouTube