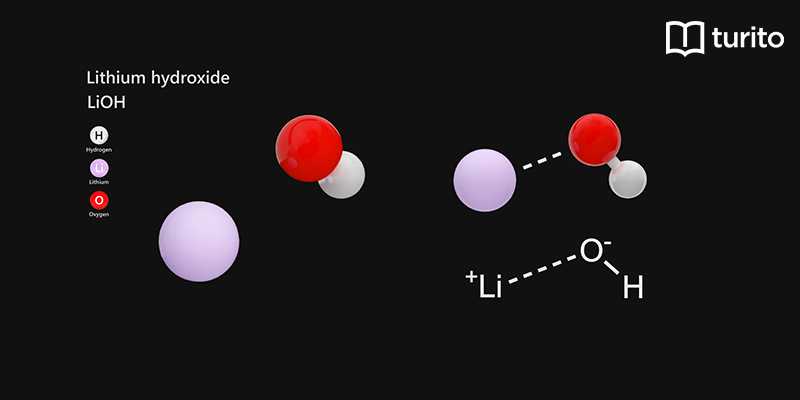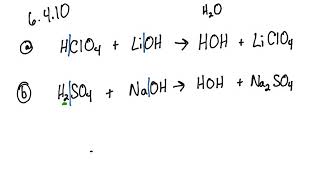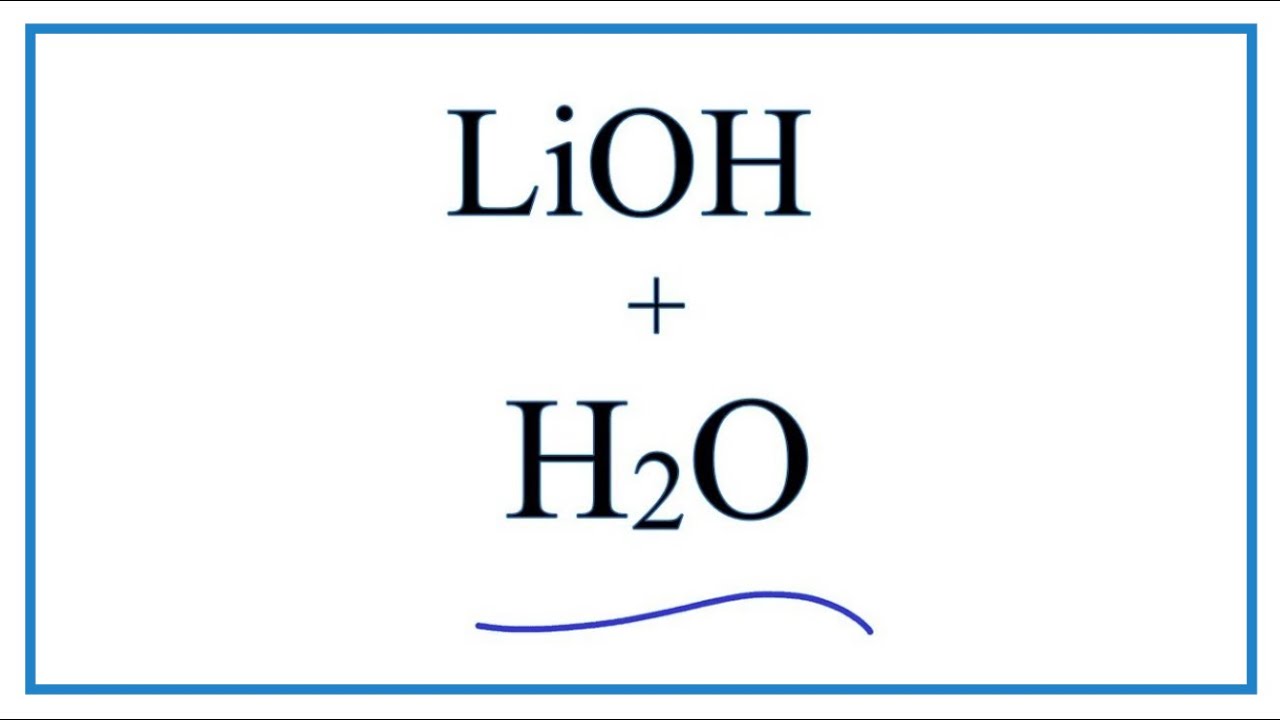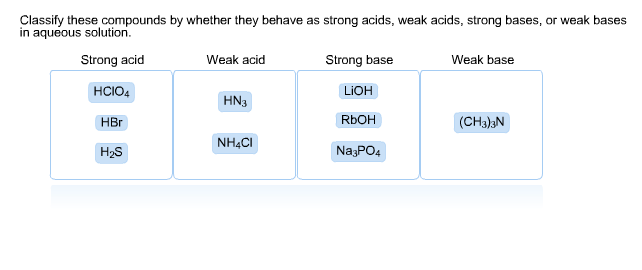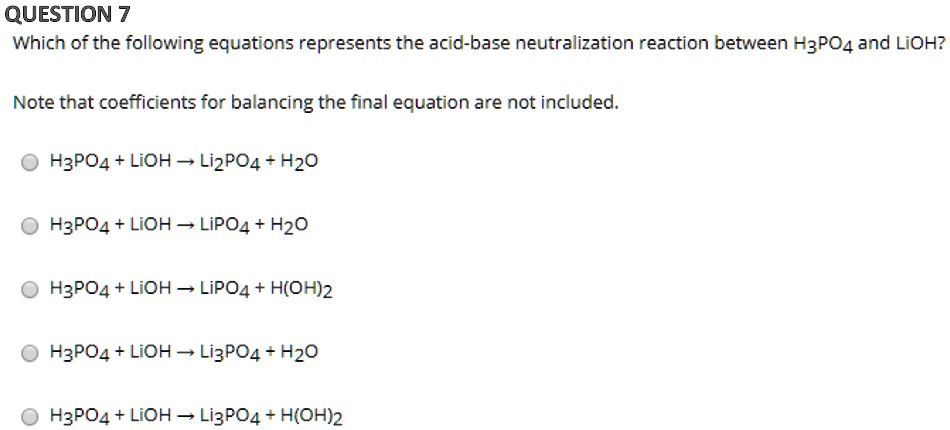
SOLVED: QUESTION 7 Which of the following equations represents the acid-base neutralization reaction between H3PO4 ad LiOH? Note that coefficients for balancing the final equation are not included: H3PO4 + LiOH LizPO4 -

Titration curves for solutions with 1.0 × 10 −4 moles of LiOH·H 2 O and... | Download Scientific Diagram
Equilibrium structures of protonated bases LiOH 2 + , NaOH 2 + , KOH 2... | Download Scientific Diagram

LiOH·H2O as a novel dual activation catalyst for highly efficient and easy synthesis of 1,3-diaryl-2-propenones by Claisen–Schmidt condensation under mild conditions - ScienceDirect



![PDF] LiOH.H2O as a catalyst for Knoevenagel and Gewald reactions | Semantic Scholar PDF] LiOH.H2O as a catalyst for Knoevenagel and Gewald reactions | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/345efc201af5c7998afa2f1e26d5b5916da3e9de/2-Table1-1.png)