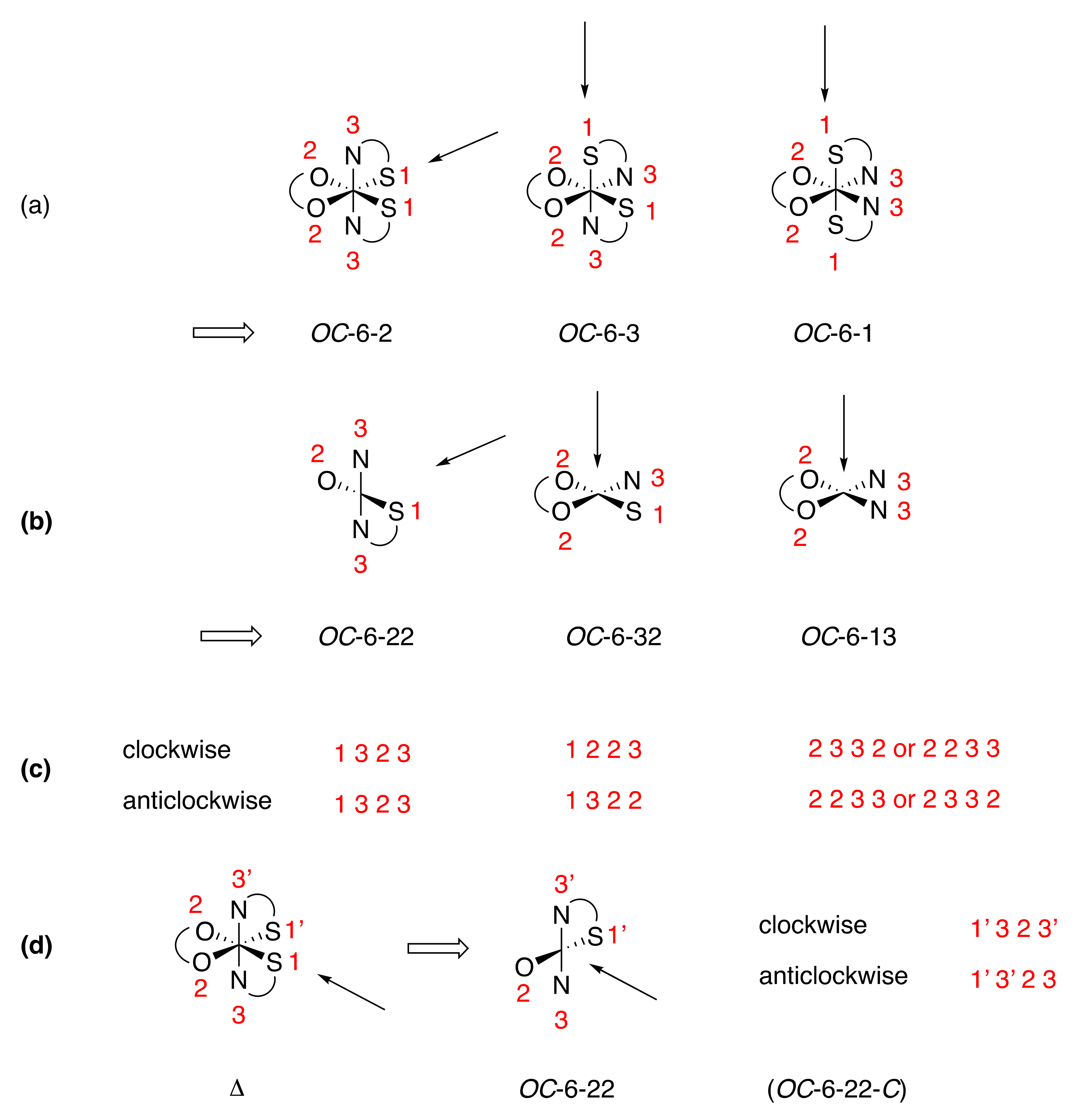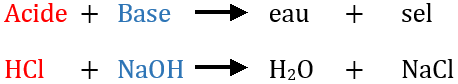
Une chimie populaire; . métaux dans la formation de sels, un métal univalent peut prendre la place de chaque atome de H, comme dans NaCl, NaN03,Na2S04; ou un métal bivalent peut prendre

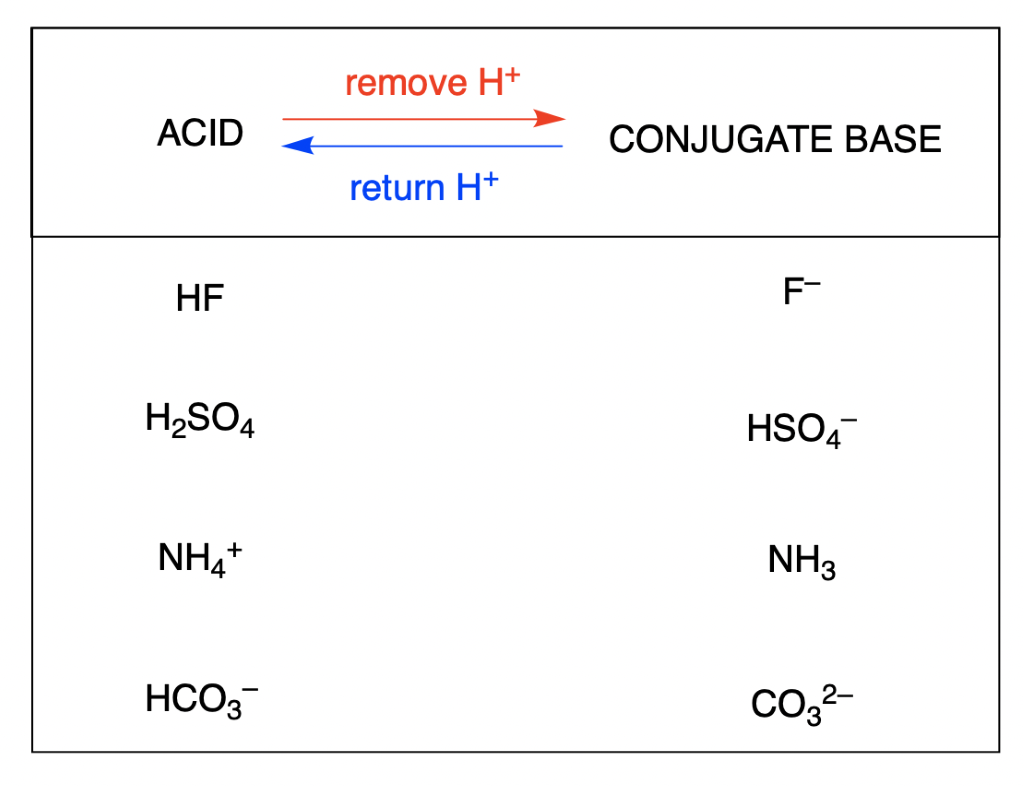
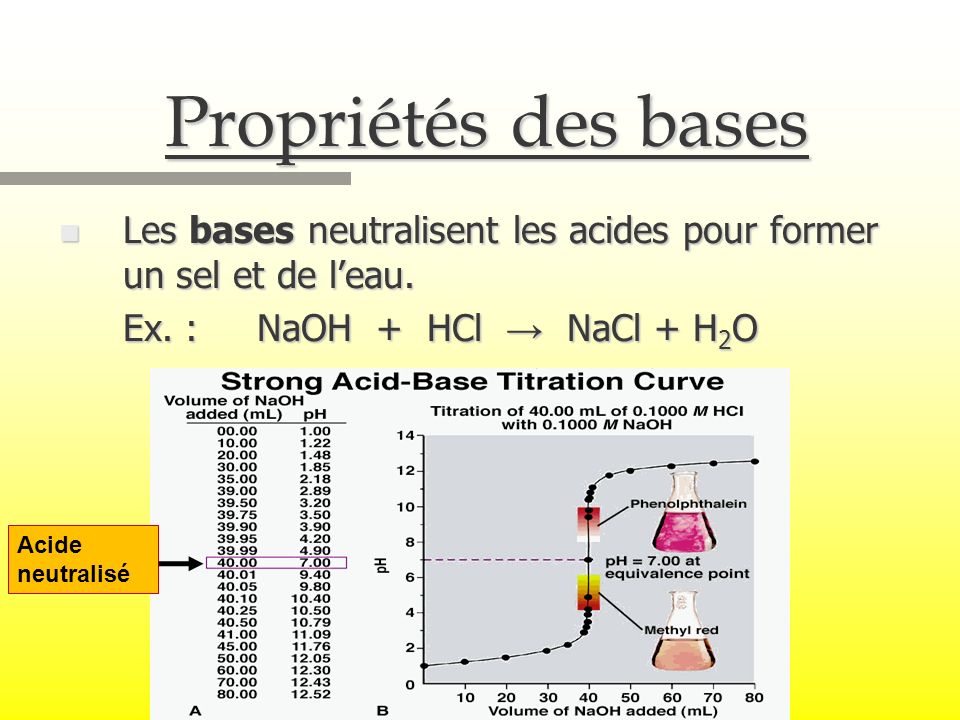
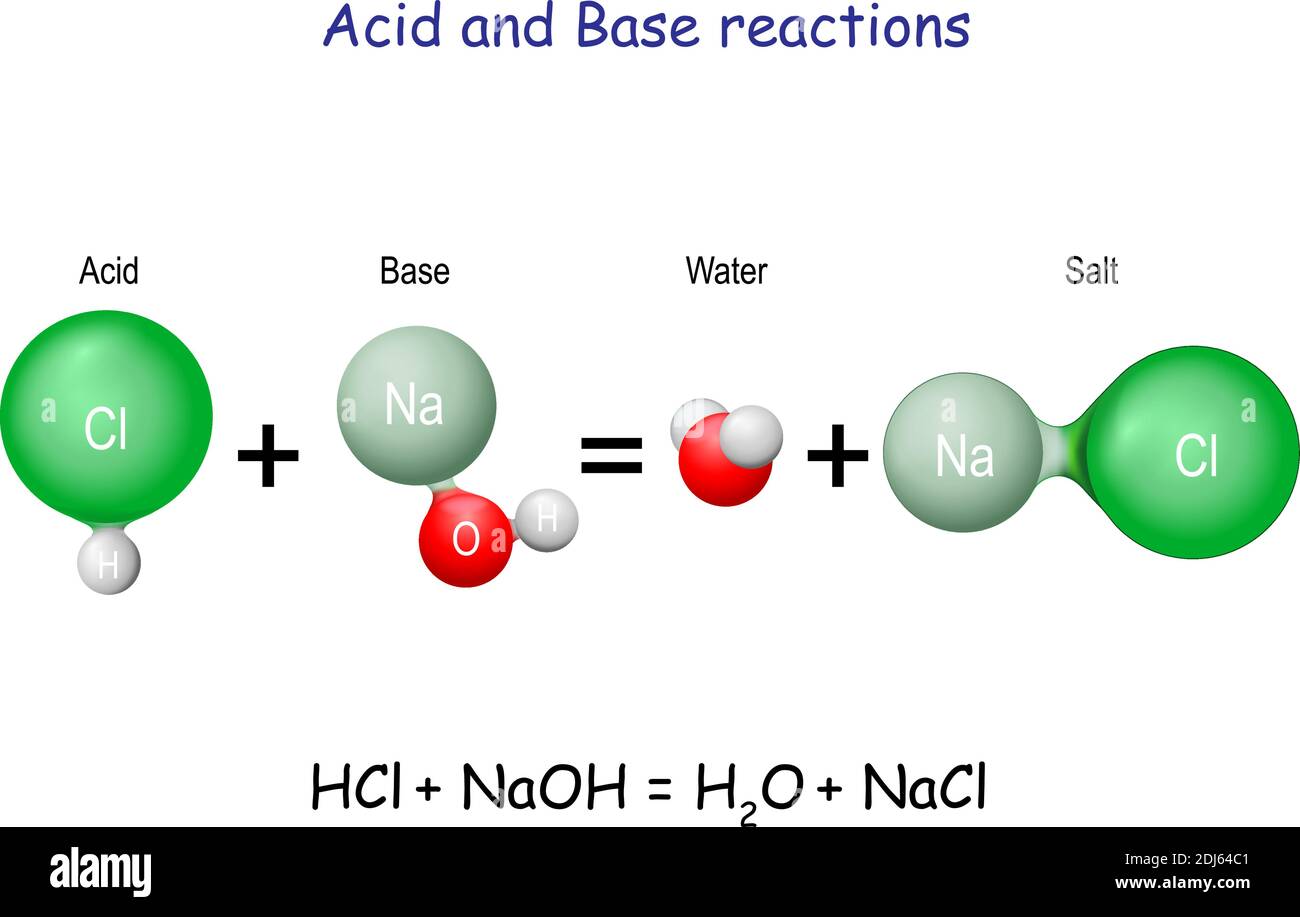
G) Sels Dans le chapitre 1, Acide + Base Sel + Eau HCl(aq) + NaOH(aq) NaCl(aq) + H2O Les sels sont des électrolytes forts qui se dissocient entièrement. - ppt video online télécharger
![PDF] The Ionization Constant of Acetic Acid in Dioxane—Water Mixtures by Herbert S. Harned, George L. Kazanjian · 10.1021/ja01301a023 · OA.mg PDF] The Ionization Constant of Acetic Acid in Dioxane—Water Mixtures by Herbert S. Harned, George L. Kazanjian · 10.1021/ja01301a023 · OA.mg](https://og.oa.mg/The%20Ionization%20Constant%20of%20Acetic%20Acid%20in%20Dioxane%E2%80%94Water%20Mixtures.png?author=%20Herbert%20S.%20Harned,%20George%20L.%20Kazanjian)
PDF] The Ionization Constant of Acetic Acid in Dioxane—Water Mixtures by Herbert S. Harned, George L. Kazanjian · 10.1021/ja01301a023 · OA.mg

Relationship between instaneous CO2 assimilation rate (A) and somatal... | Download Scientific Diagram

Methyl-esterified 3-hydroxybutyrate oligomers protect bacteria from hydroxyl radicals | Nature Chemical Biology